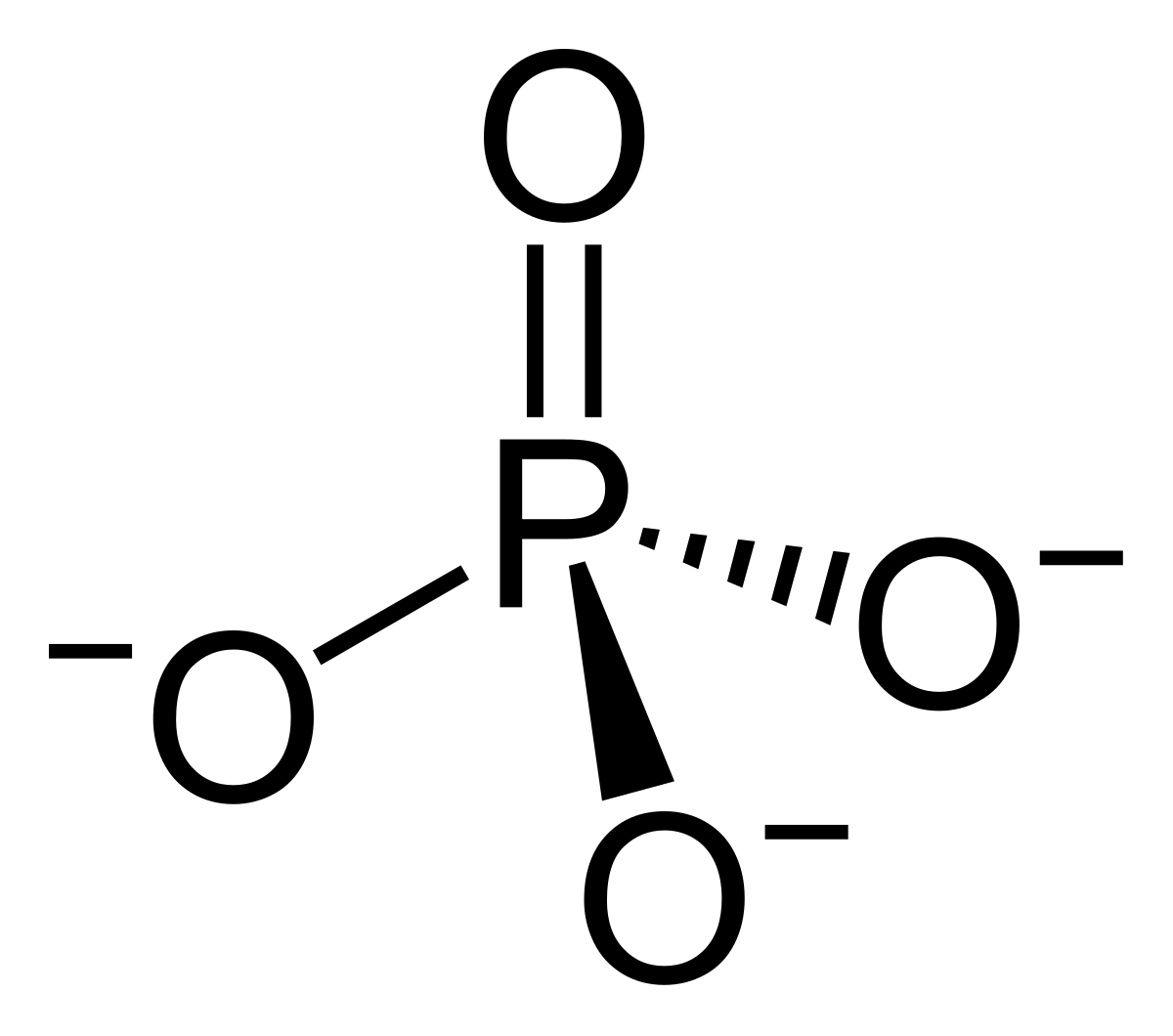
 In mineralogy and geology, phosphate refers to a rock or ore containing phosphate ions. Inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry.
In mineralogy and geology, phosphate refers to a rock or ore containing phosphate ions. Inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry. Phosphate is necessary for the formation of bones and teeth. Phosphate is also used as a building block for several important substances, including those used by the cell for energy, cell membranes, and DNA (deoxyribonucleic acid). The body obtains phosphate from foods.
Phosphate is necessary for the formation of bones and teeth. Phosphate is also used as a building block for several important substances, including those used by the cell for energy, cell membranes, and DNA (deoxyribonucleic acid). The body obtains phosphate from foods. But arguably the most useful form of phosphorus, as far as living things are concerned, is phosphates (singular: phosphate), which, simply put, are chemical compounds containing phosphorus.
But arguably the most useful form of phosphorus, as far as living things are concerned, is phosphates (singular: phosphate), which, simply put, are chemical compounds containing phosphorus.